CPS Meeting 2019
Please find informations on our final project report meeting here:
Conference report:
PDF-File (1,7 mB)
Conference website:
www.cps2019.de
1.1.3. Chemoselective reactions for the modification of proteins
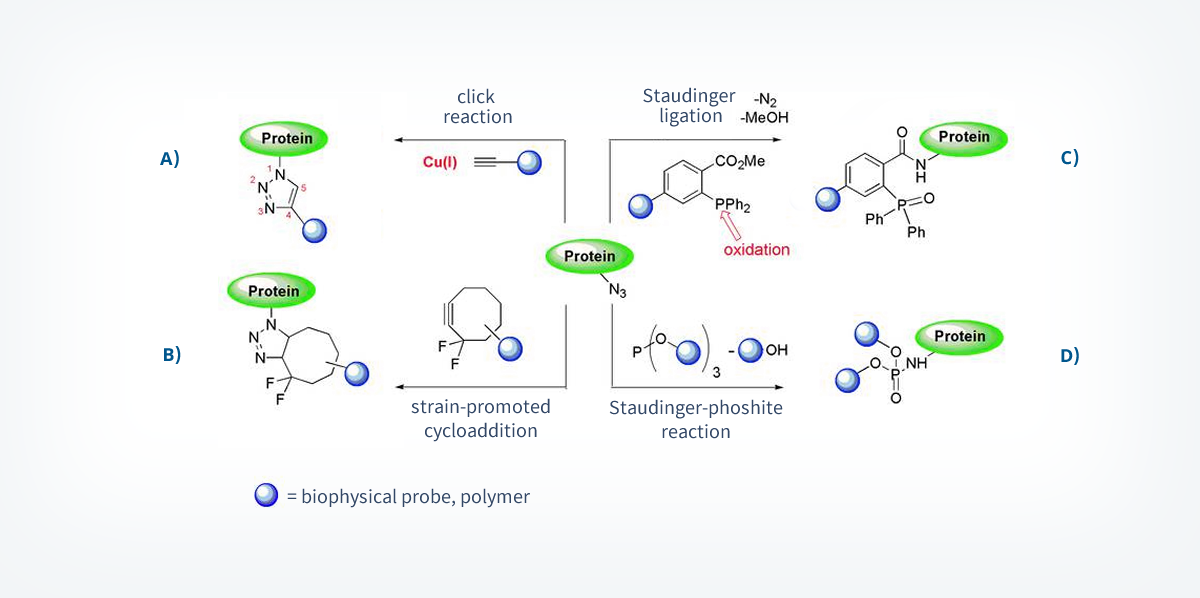
Chemoselective or bioorthogonal reactions are powerful tools that selectively react with unnatural functional groups, which can be incorporated into a protein by the methods described in chapter 1.1.2, without interfering with natural functional groups that are present in living organism.11 Additional important requirements of these reactions are, next to the chemoselectivity, high reaction and the occurrence under physiological conditions (water, high salt concentrations, reducing conditions, no toxic additives). Examples for unnatural functionalities are ketones that can be transformed to hydrazone- and oxime- conjugates, and azides, which can be applied in several chemoselective reactions. Azides are biologically inert and there are many different methods known for their incorporation into biomolecules. Currently four transformation reactions of azides fulfilling the named requirements are known and are based on dipolar cycloadditions and Staudinger-reactions.20
The most commonly used chemoselective azide-reaction is the [3+2]-dipolar cycloaddition with alkynes.21 With the help of a Cu(I) catalyst (“click-reaction”, scheme 4A)22 or by applying an energy-rich cyclooctyne-substrate (scheme 4B)23 this reaction is efficiently forming triazoles at room temperature. The Staudinger ligation is another example of a chemoselective reaction with azides (scheme 4C). This reaction is based on the transformation of phosphines to iminophosphoranes, which are then intramolecularly acetylated and act as an electrophilic trap to prevent the competitive hydrolysis of the P-N bond (Staudinger-reduction).24 Recently, a Staudinger-phosphite reaction in which a water-soluble phosphite is transformed to a phosphoramidate, was applied for the functionalization of proteins (scheme 4D).25
These transformations allowed the development of far-reaching applications in chemical biology, e.g. the visualization of active proteins in cellular systems.26, 27 However, several drawbacks limit their application.20 These drawbacks need to be addressed in the future. The toxicity of the Cu(I) catalyst hampers the use within living systems and the metal induced formation of protein-complexes changes structure and properties of the individual proteins. Cyclooctynes, which are used in metal free cycloadditions, are costly to synthesize and composed of a huge hydrophobic unit between the probe and the protein.28,29 The phosphines used in the Staudinger ligation are prone to oxidize and the kinetics of this reaction is much slower compared to the one of the cycloadditions. The Staudinger-phosphite reaction can only be applied at pH values >7. Otherwise, the hydrolysis of the phosphite to phosphonates requires a large excess of the phosphite species.
Especially by the substantial contribution of members of the priority program 1623, chemoselective reactions were successfully applied for the modifications of proteins. Examples are the glycosylation of glycoproteins by disulfide and triazol bridges.17b,30 a chemical phosphorylation by the use of the Staudinger-phosphite reaction25, a ubiquitination and SUMOylation of proteins31 and finally a conjugation of a cysteine to a maleimidocaproyl unit (MIC) by a Michael-addition. Especially the last examples should be noted, since this interdisciplinary project by researchers of the MPI Dortmund clarified the importance of the lipidation of the Ras protein family for signal transduction.33